图1 Ast、BMEC、PC12细胞鉴定(免疫荧光,×100)

3, 4-Dihydroxybenzaldehyde attenuates oxygen-glucose deprivation/reperfusion injury in brain microvascular endothelial cells and neurons by acting on astrocytes
- Academic Journal of Shanghai University of Traditional Chinese Medicine Vol. 35, Issue 4, Pages: 67-72,100(2021)
DOI:10.16306/j.1008-861x.2021.04.010
Scan for full text
Cite this article
PDF
Abstract
Objective:
To investigate the protective effects of 3, 4-dihydroxybenzaldehyde(3, 4-DD), an active component of Gastrodiae Rhizoma, on oxygen-glucose deprivation/reperfusion(OGD/R) injury in brain microvascular endothelial cells(BMEC) and neurons by acting on astrocytes(Ast), from the perspective of interconnection between neurovascular unit(NVU) cells.
Methods:
BMEC and Ast were isolated and cultured from neonatal rats.The co-culture systems of Ast and BMEC, Ast and PC12 cells were constructed by Transwell chamber, respectively.The co-culture cells were divided into the control group, model group, butyphthalide(NBP) intervention(1×10-4mol/L) group and 3, 4-DD intervention(1×10-7mol/L) group.The drug treatment groups were treated with the corresponding drug in advance.After drug intervention for 24 hours, OGD/R injury was induced in the model group and drug treatment groups.The transendothelial electrical resistance(TEER) of the cells inside Transwell chamber was measured by cell electrical resistance meter.The activity of lactate dehydrogenase(LDH) in BMEC and PC12 cells was detected by kit.The content of brain-derived neurotrophic factor(BDNF) in the medium was detected by ELISA.The mRNA expressions of Occludin in BMEC and phospholipase C-γ(Plc-γ) in PC12 cells were detected by PCR.
Results:
①Ast-BMEC co-culture system: Compared with the control group, the TEER value, LDH activity and BDNF content of the model group were significantly decreased(P<0.01), and the Occludin mRNA expression was significantly increased(P<0.01) .Compared with the model group, the TEER value, LDH activity, BDNF content and Occludin mRNA expression were significantly increased in the 3, 4-DD intervention group(P<0.01), and the LDH activity, BDNF content and Occludin mRNA expression were also significantly increased in the NBP intervention group(P<0.01) .The TEER value of 3, 4-DD intervention group was significantly higher than that of NBP intervention group(P<0.01) .②Ast-PC12 co-culture system: Compared with the control group, the TEER value, LDH activity and BDNF content of the model group were significantly decreased(P<0.01), and the Plc-γ mRNA expression was significantly increased(P<0.01) .Compared with the model group, the LDH activity, BDNF content and Plc-γ mRNA expression were significantly increased in the 3, 4-DD intervention group(P<0.05, P<0.01), and the LDH activity and Plc-γ mRNA expression were also significantly increased in the NBP intervention group(P<0.01) .
Conclusion:
3, 4-DD can promote the release of BDNF by Ast, act on BMEC and neurons, maintain intercellular structure, enhance synaptic plasticity of neurons, and then alleviate OGD/R injury.
脑缺血再灌注损伤(cerebral ischemic reperfusion injury,CIRI)导致神经血管单元(neurovascular unit,NVU)中的脑微血管内皮细胞(brain microvascular endothelial cell,BMEC)、胶质细胞受损,血脑屏障(blood brain barrier,BBB)受到破坏,神经元凋亡,进而出现神经功能障碍[
1 材料与方法
1.1 实验材料
1.1.1 动物
SD大鼠,SPF级,体质量250~300 g,购自辽宁长生生物技术股份有限公司,生产许可证号:SCXK(辽)2015-0001,合格证号:211002300040630。所有动物均饲养于云南中医药大学实验动物中心,饲养室温度22~24℃,湿度50%~60%,12 h明暗交替,可自由进食、饮水。各项实验操作均获得云南中医药大学动物保护委员会批准(批准号:R-06202003)。
取成年雌性SD大鼠1只,与2只雄鼠进行合笼饲养,第2天观察鼠笼底层是否有阴栓,出现阴栓之日起将雌鼠与雄鼠分笼饲养。
1.1.2 细胞
PC12细胞,由中国科学院上海生命科学研究院提供。
1.1.3 药物与试剂
3,4-DD(货号:P13N),英国Matrix Scientific公司;NBP(货号:118170230),石药集团恩必普药业有限公司。
0.25%Trypsin-EDTA试液(货号:03-050-1A)、特级胎牛血清(fetal bovine serum,FBS,货号:04-01-1A),以色列Biological Industries公司;明胶(货号:5016),天津光复精细化工研究所;多聚赖氨酸(货号:100502),美国MP Biomedicals公司;牛血清白蛋白(bovine serum albumin,BSA,货号:033100g),美国Amresco公司;DMEM/F12培养基(货号:CLL330500B)、DMEM/RPMI 1640培养基(货号:58903),美国Gibco公司;青霉素-链霉素双抗试液(货号:030311B),美国Hyclone公司;D-Hank’s试液(货号:H104)、山羊血清(货号:SL2-1010ML),北京索莱宝科技有限公司。
兔抗Ⅷ因子抗体(货号:NB10091761Cy),美国Novus Biologicals公司;兔抗胶质细胞原纤维酸性蛋白(glial fibrillary acidic protein,GFAP)抗体(货号:Ab3546)、兔抗MAP2抗体(货号:Ab0453),英国Abcam公司;山羊抗兔荧光二抗(货号:C2306),美国Sigma公司;DAPI试剂(货号:F6057-20ML),北京索莱宝科技有限公司;甲醇(货号:2013802),上海化学试剂有限公司;乳酸脱氢酶(lactate dehydrogenase,LDH)检测试剂盒(货号:A020-2)、脑源性神经营养因子(brain-derived neurotrophic factor,BDNF)ELISA检测试剂盒(货号:H069),南京建成生物工程研究所;TRIzol试剂(货号:191012)、PrimeScriptTM RT Master Mix试剂盒(货号:AGH1533A),日本TaKaRa公司;PowerUpTM SYBRTM Green Master Mix试剂盒(货号:662399),美国Thermo Fisher Scientific公司。
1.1.4 主要仪器
CO2细胞培养箱(型号:3111),美国Thermo公司;生物安全柜(型号:BSC-1300ⅡA2),苏州安泰空气技术有限公司;高速离心机(型号:KA-1000),上海安亭科学仪器厂;电子分析天平(型号:AB204-S),瑞士Mettler Toledo公司;分析天平(型号:XS125A),瑞士Precisa公司;倒置相差显微镜(型号:Ti-S),日本Nikon公司;数显恒温水浴锅(型号:HH-S28),北京市华仁医疗设备公司;酶标仪(型号:Infinite M200 Pro),瑞士Tecan公司;细胞电阻仪(型号:Millicell ERS-2)、Transwell小室(孔径0.4 μm,有效膜面积0.33 cm2),美国Millipore公司;实时荧光定量PCR仪(型号:QuantStudio 5),美国Thermo Fisher Scientific公司。
1.2 细胞培养
1.2.1 PC12细胞培养
复苏PC12细胞,加入含10%FBS、40 U/ml青霉素-链霉素双抗试液的DMEM/RPMI 1640培养基,置于37℃、5%CO2、饱和湿度的细胞培养箱,每2 d换液1次,待细胞融合至80%~90%进行传代培养。
1.2.2 Ast分离与培养
取出生后第5天的乳鼠,分离大脑皮质,用无菌组织剪快速将其剪碎,离心(1 000 r/min,3 min),弃除上清液,以Trypsin-EDTA试液作用5 min进行消化,再加入含20%FBS、40 U/ml青霉素-链霉素双抗试液的DMEM/F12培养基终止消化,组织碎片用滤网进行过滤,将过滤后的混悬液离心(1 000 r/min,5 min),收集管底细胞沉淀,接种于0.01%多聚赖氨酸包被的培养瓶中,置于37℃细胞培养箱,24 h后换入新鲜培养液,细胞生长状态稳定后每2 d更换1次培养液。待细胞生长密度达90%,以摇瓶法纯化细胞(480 r/min,8 h),纯化后的细胞用于后续实验。
1.2.3 BMEC分离与培养
取出生后第7天的乳鼠,分离大脑皮质,用无菌组织剪将组织剪碎,离心(1 000 r/min,3 min),弃除上清液后加入BSA(体积比1∶1混合),充分吹打、混匀,离心(2 500 r/min,8 min),收集管底血管段,接种于明胶包被的培养瓶中(内有含20%FBS、40 U/ml青霉素-链霉素双抗试液的DMEM/F12培养基),置于37℃细胞培养箱,24 h后换入新鲜培养液,细胞生长状态稳定后每2 d更换1次培养液。待细胞生长密度达90%,进行传代及纯化,第3代细胞用于实验。
1.2.4 Ast-BMEC和Ast-PC12共培养模型[
取对数生长期细胞,调整细胞密度(PC12细胞1×106个/ml、BMEC细胞4×105个/ml),以每孔2 ml的细胞悬液接种于6孔板板底,分别设置4个复孔。待两种细胞生长2 d后,调整Ast密度为2.5×105个/ml,接种于已包被多聚赖氨酸的Transwell小室内侧。待Ast贴壁2 h后,将Ast加入分别接种PC12和BMEC的6孔板,继续培养,以复制Ast-BMEC和Ast-PC12共培养模型。
1.3 药物干预与OGD/R模型复制
按“1.2.4”项下分别构建Ast-BMEC和Ast-PC12共培养体系,将共培养细胞分为对照组、模型组、NBP干预组、3,4-DD干预组,每组均设置4个复孔。NBP干预组和3,4-DD干预组预先给予相应药物干预,药物浓度设置依据前期药效学研究[
1.4 检测项目与方法
1.4.1 免疫荧光鉴定
分别取“1.2”项下制备的PC12、Ast、BMEC细胞,用预冷的PBS清洗5 min,重复3次;每孔加入4%多聚甲醛500 μl,室温固定1 h;PBS清洗5 min,重复3次;再加入0.1%Triton X-100试液500 μl,室温孵育20 min,以增加板底细胞的通透性;PBS清洗5 min,重复3次;PC12细胞加入MAP2抗体(1∶500)、Ast加入GFAP抗体(1∶200)、BMEC加入Ⅷ因子抗体(1∶300),4℃封闭过夜。次日先以PBS清洗5 min,重复3次;再分别加入荧光二抗(按体积稀释比例1∶100干预PC12细胞,按体积稀释比例1∶200干预Ast和BMEC)室温下封闭2 h;PBS清洗5 min,重复3次;后加入DAPI试液,室温孵育15 min,抗荧光淬灭剂封片。荧光显微镜下观察抗体的阳性表达情况。
1.4.2 ELISA检测BDNF表达
按“1.3”项进行细胞分组与干预,取板底细胞培养基,参照试剂盒说明书的操作步骤,检测各组细胞分泌的BDNF含量。
1.4.3 试剂盒检测LDH活性
按“1.3”项进行细胞分组与干预,取板底细胞进行裂解,参照试剂盒说明,检测各组细胞LDH活性。酶标仪测定450 nm处吸光度(OD值),计算LDH活性(U/g)=(测定OD值-对照OD值)÷(标准OD值-空白OD值)×标准浓度(2 μmol/L)÷待测样本蛋白浓度(g/ml)。
1.4.4 跨内皮电阻(transendothelial electrical resistance,TEER)值测定
按“1.3”项进行细胞分组与干预,采用细胞电阻仪测定各组Transwell小室内侧细胞的TEER值,每个小室测量3次,计算TEER(Ω·cm2)=(TEER测定-TEER对照)×有效膜面积(0.33 cm2)。
1.4.5 PCR检测相关基因表达
按“1.3”项进行细胞分组与干预,取板底细胞,PBS清洗5 min,重复3次;每孔加入TRIzol试剂1 ml充分裂解共培养体系中BMEC或PC12细胞,加入0.2 ml氯仿提取总RNA,应用PrimeScriptTM RT试剂盒进行逆转录反应合成cDNA。以GAPDH为内参进行荧光定量PCR扩增,以检测各组细胞中Occludin和Plc-γ的mRNA表达水平。扩增引物由昆明硕擎生物技术有限公司合成,引物序列见
| 基因名称 | 引物序列 |
|---|---|
| GAPDH | 上游:5′-GATGGTGAAGGTCGGTGTGA-3′ |
| 下游:5′-AACTTGCCGTGGGTAGAGTC-3′ | |
| Occludin | 上游:5′-CGGCGCAAAAGGAAGGAATC-3′ |
| 下游:5′-AGAAGTGCGGGTATGCTCTG-3′ | |
| Plc-γ | 上游:5′-TACTCTACCGCACCCGGTTA-3′ |
| 下游:5′-CGTACAGTTTCAGCATGGCG-3′ |
1.5 统计学方法
采用SPSS 20.0软件进行统计学分析。计量资料以 ±s表示,多组间比较采用单因素方差分析,两组间比较采用LSD-t检验。以P<0.05为差异有统计学意义。
2 结果
2.1 免疫荧光鉴定细胞
免疫荧光检测显示,Ast细胞GFAP阳性表达呈红色,BMEC细胞Ⅷ因子阳性表达呈红色,PC12细胞MAP2阳性表达呈绿色,实验所用细胞纯度均达98%以上。见
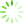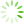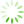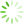
2.2 对Ast-BMEC共培养体系的影响
在Ast-BMEC共培养体系,与对照组相比,模型组TEER值、LDH活性、BDNF含量显著降低(P<0.01),Occludin mRNA表达显著增加(P<0.01);与模型组相比,3,4-DD干预组TEER值、LDH活性、BDNF含量以及Occludin mRNA表达均显著升高(P<0.01),NBP干预组LDH活性、BDNF含量及Occludin mRNA表达亦显著增加(P<0.01);与NBP干预组相比,3,4-DD干预组TEER值明显升高(P<0.01)。结果提示:3,4-DD通过作用Ast减轻OGD/R诱导的细胞损伤,增加BMEC间紧密连接蛋白Occludin mRNA表达,使受损BMEC间的紧密连接重组,进而保护BBB。见
| 组别 | TEER(Ω·cm2) | LDH(U/g) | BDNF(ng/L) | Occludin |
|---|---|---|---|---|
| 对照组 | 327.50±36.63 | 3.00±0.13 | 6.93±0.40 | 1.00±0.00 |
| 模型组 | 256.80±21.20## | 2.08±0.28## | 4.23±0.71## | 11.06±1.26## |
| NBP干预组 | 239.00±16.57 | 2.92±0.17** | 6.42±0.70** | 16.43±1.90** |
| 3,4-DD干预组 | 325.00±10.42**△△ | 3.15±0.26** | 6.48±0.56** | 15.39±0.77** |
注: 与对照组比较,##P<0.01;与模型组比较,**P<0.01;与NBP干预组比较,△△P<0.01
2.3 对Ast-PC12共培养体系的影响
在Ast-PC12共培养体系,与对照组相比,模型组TEER值、LDH活性、BDNF含量显著降低(P<0.01),Plc-γ mRNA表达显著增加(P<0.01);与模型组相比,3,4-DD干预组LDH活性、BDNF含量及Plc-γ mRNA表达显著升高(P<0.05,P<0.01),NBP干预组LDH活性及Plc-γ mRNA表达亦显著升高(P<0.01);两干预组组间比较,差异均无统计学意义(P>0.05)。结果提示:3,4-DD通过作用Ast,增加神经元轴突膜蛋白Plc-γ mRNA表达,调节突触的可塑性,恢复神经元间信号的传导,减轻神经元损伤。见
| 组别 | TEER(Ω·cm2) | LDH(U/g) | BDNF(ng/L) | Plc-γ |
|---|---|---|---|---|
| 对照组 | 321.30±16.74 | 3.68±0.17 | 11.58±1.55 | 1.00±0.00 |
| 模型组 | 251.30±22.35## | 2.63±0.36## | 6.73±1.01## | 19.83±1.40## |
| NBP干预组 | 269.80±27.61 | 3.43±0.28** | 9.50±0.59 | 25.22±1.76** |
| 3,4-DD干预组 | 265.50±26.89 | 3.28±0.25* | 10.30±0.74* | 24.04±1.88** |
注: 与对照组比较,##P<0.01;与模型组比较,*P<0.05,**P<0.01
3 讨论
CIRI是导致缺血性脑卒中(ischemic stroke,IS)病情恶化的重要原因,CIRI形成后,一方面BBB通透性改变、胶质细胞增生等一系列复杂的病理变化诱使神经元变性、坏死[
CIRI时,减轻BBB损伤、保证其屏障功能的完整性是维持NVU稳态的前提,BBB结构重塑并稳定后,将为受损神经元的修复提供有益的脑微环境[
本研究观察了3,4-DD干预对共培养体系细胞TEER值、LDH活性以及下层BMEC紧密连接蛋白Occludin mRNA表达的影响,结果表明:3,4-DD可作用于Transwell小室上层的Ast,诱使LDH活性升高,并上调小室下层BMEC中Occludin mRNA表达,3,4-DD亦促使细胞TEER值显著增加,提示3,4-DD通过作用Ast减轻OGD/R诱导的细胞损伤,增加BMEC间紧密连接蛋白Occludin mRNA表达,使受损BMEC间的紧密连接重组,进而起到保护BBB的作用。
Ast和BMEC不仅是BBB的结构基础,还可共同为神经元提供BDNF[
本研究选择了分化型且具有类神经元表达特性的PC12细胞株作为神经元的替代细胞[
综上,3,4-DD通过促进Ast释放BDNF作用于BMEC和神经元,维持细胞间结构,增强神经元突触可塑性,进而减轻OGD/R损伤。
参考文献:
ABE K. Neurological diseases and neurovascular unit(NVU)[J]. Nihon Rinsho,2014,72(4):594-599. [Baidu Scholar]
VANGILDER R L,ROSEN C L,BARR T L,et al. Targeting the neurovascular unit for treatment of neurological disorders[J]. Pharmacol Ther,2011,130(3):239-247. [Baidu Scholar]
张扬.缺血性脑卒中恢复期神经血管单元的治疗靶点研究[D].南京:南京医科大学,2016. [Baidu Scholar]
RANSOM B R,RANSOM C B. Astrocytes:multitalented stars of the central nervous system[J]. Methods Mol Biol,2012,814:3-7. [Baidu Scholar]
何芳雁.天麻对MCAO/R模型大鼠血脑屏障的保护作用及机制研究[D].昆明:云南中医学院,2015. [Baidu Scholar]
贾媛媛.天麻酚性成分促血管新生及神经修复的作用研究[D].昆明:云南中医学院,2017. [Baidu Scholar]
蒋石.天麻酚性成分对脑缺血致脑内炎症损伤的保护作用及机制研究[D].昆明:云南中医学院,2015. [Baidu Scholar]
颜汉文.天麻酚性成分对神经血管单元的保护作用[D].昆明:云南中医学院,2017. [Baidu Scholar]
薛强.“脑神经血管单元”体外模型的成模性研究[D].重庆:西南大学,2013. [Baidu Scholar]
刘抒雯,杨丽华,马春,等.中医药保护脑缺血再灌注损伤后神经血管单元的作用[J].中国实验方剂学杂志,2018,24(23):225-234. [Baidu Scholar]
ROUX P,ESQUENAZI S. Astrocytes mediate cerebral cortical neuronal axon and dendrite growth,in part,by release of fibroblast growth factor[J]. Neurol Res,2002,24(1):81-92. [Baidu Scholar]
HOUGAARD A,AMIN F M,CHRISTENSEN C E,et al. Increased brainstem perfusion,but no blood-brain barrier disruption,during attacks of migraine with aura[J]. Brain,2017,140(6):1633-1642. [Baidu Scholar]
NAKAGAWA S,DELI M A,NAKAO S,et al. Pericytes from brain microvessels strengthen the barrier integrity in primary cultures of rat brain endothelial cells[J]. Cell Mol Neurobiol,2007,27(6):687-694. [Baidu Scholar]
冯岚,陈德伟,高文祥,等.7种不同组织来源细胞缺氧损伤效应评价[J].第三军医大学学报,2019,41(6):570-574. [Baidu Scholar]
MURER M G,BOISSIERE F,YAN Q,et al. An immunohistochemical study of the distribution of brain-derived neurotrophic factor in the adult human brain,with particular reference to Alzheimer’s disease[J]. Neuroscience,1999,88(4):1015-1032. [Baidu Scholar]
ANDRESKA T,AUFMKOLK S,SAUER M,et al. High abundance of BDNF within glutamatergic presynapses of cultured hippocampal neurons[J]. Front Cell Neurosci,2014,8:107. [Baidu Scholar]
NUMAKAWA T,ODAKA H,ADACHI N. Actions of Brain-Derived Neurotrophin Factor in the Neurogenesis and Neuronal Function,and Its Involvement in the Pathophysiology of Brain Diseases[J]. Int J Mol Sci,2018,19(11):3650. [Baidu Scholar]
ZHANG H T,ZHANG P,GAO Y,et al. Early VEGF inhibition attenuates blood-brain barrier disruption in ischemic rat brains by regulating the expression of MMPs[J]. Mol Med Rep,2017,15(1):57-64. [Baidu Scholar]
APPELT-MENZEL A,CUBUKOVA A,GÜNTHER K,et al. Establishment of a Human Blood-Brain Barrier Co-culture Model Mimicking the Neurovascular Unit Using Induced Pluri-and Multipotent Stem Cells[J]. Stem Cell Reports,2017,8(4):894-906. [Baidu Scholar]
120
Views
455
Downloads
0
CSCD
2
CNKI Cited
Related Articles
Related Author
Related Institution
